CRII: CPS: Wearable-Machine Interface Architectures
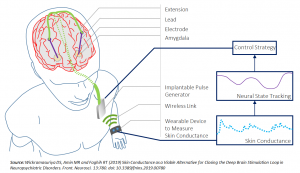
Wrist-worn wearable devices provide rich sets of pulsatile physiological data under various modalities and circumstances. An unexploited capability is that the pulsatile physiological time series collected by wrist-worn wearable devices can be used for recovering internal brain dynamics. The goal of this project is to present wearable machine-interface (WMI) architectures related to mental stress and their potential applications for tracking fatigue and arousal states. Decoding brain states using wrist-worn wearables will transform how mental-stress-related diseases are diagnosed and treated. This proposal presents two design classes of WMI architectures related to mental stress: (1) A decoder that using skin conductance data recovers undesired stimuli triggered brain activity from wearables and a controller that delivers intermittent stimulation to reverse the adverse effect of stimuli. (2) A decoder that using cortisol data recovers undesired stimuli-triggered inhibition of cortisol secretion from wearables and a controller that delivers intermittent stimulation to generate the desired cortisol profile. The proposed methods will be validated by analyzing electrodermal activity as well as concurrent cortisol and adrenocorticotropic hormone pulsatile data in the context of mental-stress-related arousal and fatigue.
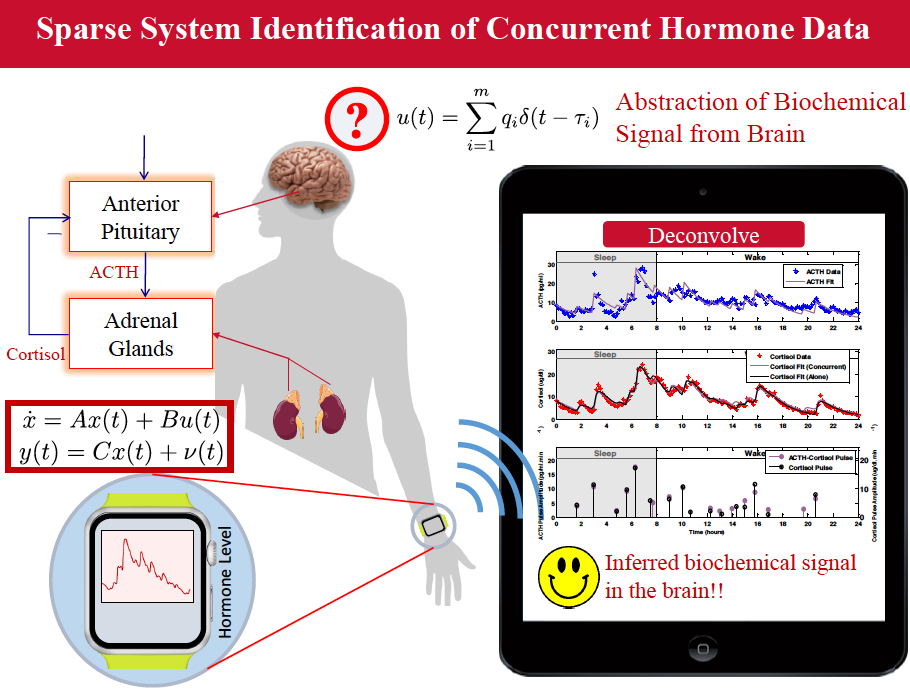
Signal Processing Framework that Empowers Inferring Unobservable Hypothalamic Secretory Events from Neuroendocrine Data
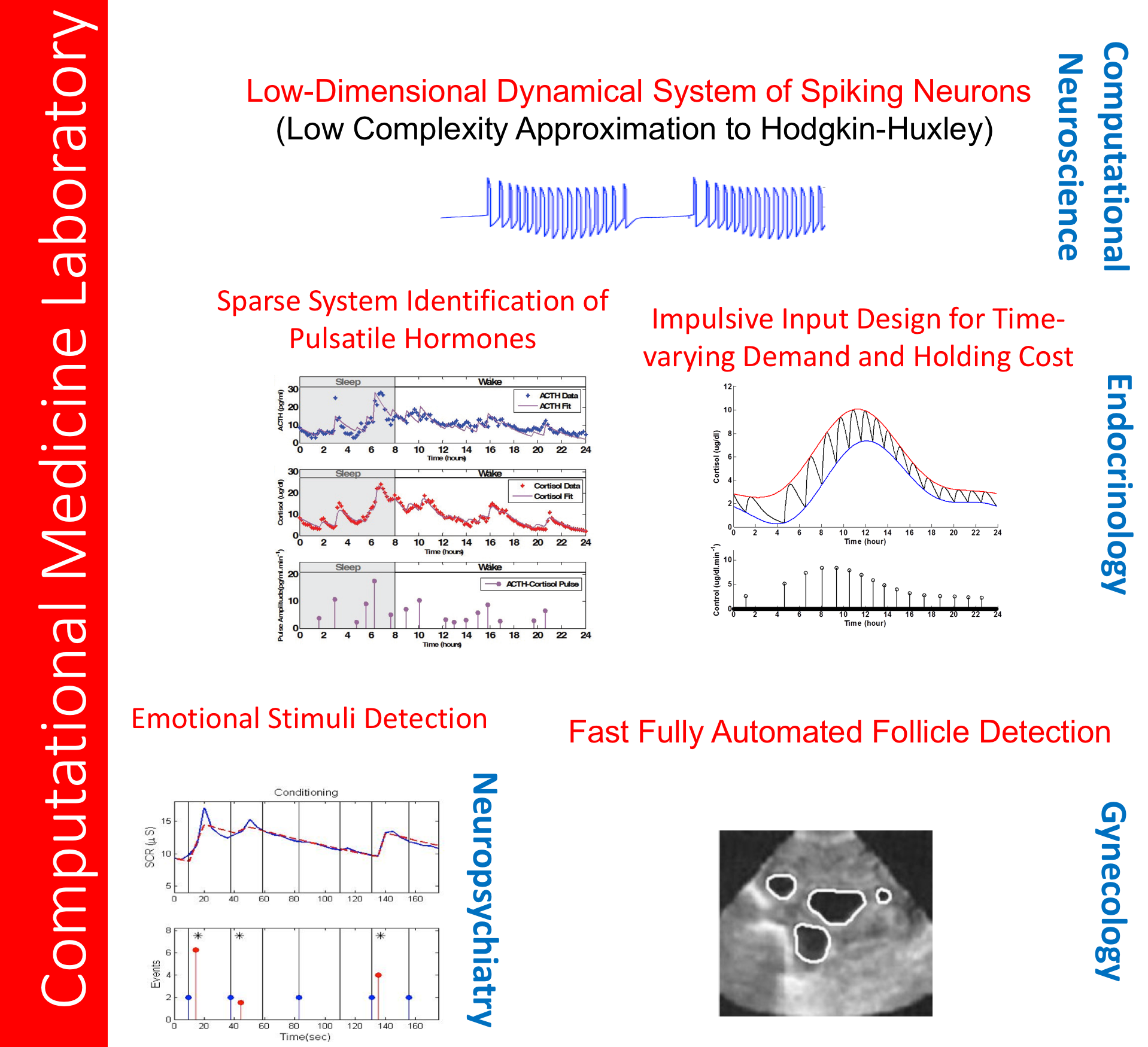
We have pioneered a framework that combines a minimal physiological model with compressed sensing techniques from signal processing and generalized cross validation from statistics to answer an important question in neuroendocrine data analysis, namely, determining the timing and amplitude of secretory events in neuroendocrine data. This framework can also be used for analysis of concurrent measurements of pairs of pulsatile endocrine hormones whose interactions are controlled through feedback loops. Moreover, this framework can be used to investigate pathological neuroendocrine states. For example, the dynamics underlying the pulsatile release of cortisol in response to corticotropin releasing hormone (CRH) from the hypothalamus and adrenocorticotropic hormone (ACTH) from the pituitary are not well understood. In particular, recovering both the timing and magnitude of cortisol secretions as well as that of ACTH from periodic serum cortisol and ACTH measurements is a challenge. The physiology is first modeled using ordinary differential equations with parameters to account for the cortisol infusion rate into the bloodstream and clearance by the liver. Pituitary dynamics governing ACTH release can be modeled similarly. Exploiting the sparsity of secretory events, a combination of compressed sensing techniques from signal processing and generalized cross validation from statistics is applied to obtain cortisol and ACTH secretions. The same approach can also be utilized for solely recovering cortisol secretions from measurements. Agreement with known physiology illustrates the ability of the model to analyze the HPA-axis as a whole and lead to optimal clinical treatment procedures for cortisol-related disorders.
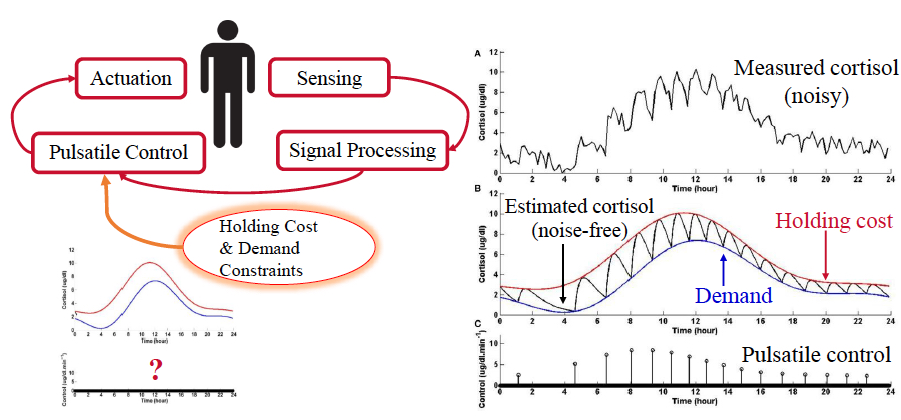
An Optimization Formulation that Enables Mathematical Characterization of Physiological Pulsatile Control
Cortisol, the body’s primary stress hormone, helps regulate metabolism and increases blood sugar concentration in response to stimuli. Cortisol is secreted by the adrenal glands in the form of pulses. This release of cortisol into the bloodstream is in response to secretory events from the hypothalamus and the pituitary. It is postulated that the pituitary gland is solving an optimal control problem so as to minimize the number of pulsatile secretions in order to conserve energy. Furthermore, the concentration of cortisol needs to be maintained within the limits of time-varying upper and lower bounds throughout the day. We have devised an optimization formulation that characterizes the pulsatile control underlying secretion of pulsatile endocrine hormones. The problem is formulated as an L0-norm cost function minimization and is solved using a re-weighted L1-norm minimization for improved time complexity. Physiologically plausible solutions that are in agreement with known daily rhythms of blood cortisol indicate the possibility of developing pulsatile controllers for neuroscience applications. Consider for instance, a case where a patient with cortisol deficiency is administered a few doses of cortisone each day. Ideally, such a course of treatment would be replaced by a bio-inspired optimal impulse controller emulating actual physiological regulation. This formulation can be used to optimally treat some endocrine disorders. This type of bio-inspired pulsatile controller can also be used in brain-machine interface architectures in order to reduce the brain implant’s energy consumption.
Automated Ovarian Follicular Monitoring via Hybrid Image Segmentation
The accuracy of computerized ovarian follicle detection methods is often hampered by noise and the close proximity of follicles to each other. Moreover, both fully and semi-automated segmentation approaches are often too slow to have clinical applicability. Here, a novel methodology is presented whereby noise is suppressed using Singular-Value Decomposition (SVD) followed by Anisotropic Diffusion. A combination of segmentation schemes (active contours, distance-regularized level sets and watershed segmentation) are applied to ultrasound images based on intrinsic image properties such as pixel level intensity and characteristics of the follicle regions detected. The method permits rapid ovarian follicle segmentation with the capability of differentiating between closely-spaced follicles and an accurate distinction between follicles and walls of the ovary. Given the variability among clinicians and significant time requirement in identifying follicles, this is a promising completely-automated approach for estimating ovarian reserve and evaluating fertility treatment.

A Signal Processing Framework that Empowers Characterization of Neuropsychiatric States
We have developed a low-dimensional model for understanding one’s emotional and cognitive states from wrist-worn wearable data (e.g. skin conductance response), brain recordings (e.g. spiking activity). This is essential for characterizing one’s neuropsychiatric state. For example, in we have recovered fear stimuli by investigating skin conductance data in a fear conditioning study. Fear conditioning refers to a basic phenomenon in which a person learns to anticipate an unpleasant future event. In the prototypical experimental setup used to study its occurrence, a conditioned stimulus (e.g. a light, tone or geometric shape) is presented to a participant followed by an aversive stimulus such as an electric shock, an unpleasant loud noise or a human scream. Eventually, the conditioned stimulus alone begins eliciting a fear response, and is typically measured by electrodermal activity. We have proposed an ordinary differential model of nerve activity of the sweat glands and by utilizing the sparsity of the electric shocks, we have recovered the conditioned and unconditioned stimuli. Time delays between the visual cues and the fear responses when the conditioned stimuli are presented without actually providing a shock provide interesting insight into how participants re-learn the original unpleasant association during the extinction phase. Our goal is to extend this study to dynamically track emotional states and perhaps develop therapeutic measures for treating accompanying disorders such as Post-Traumatic Stress Disorder (PTSD).
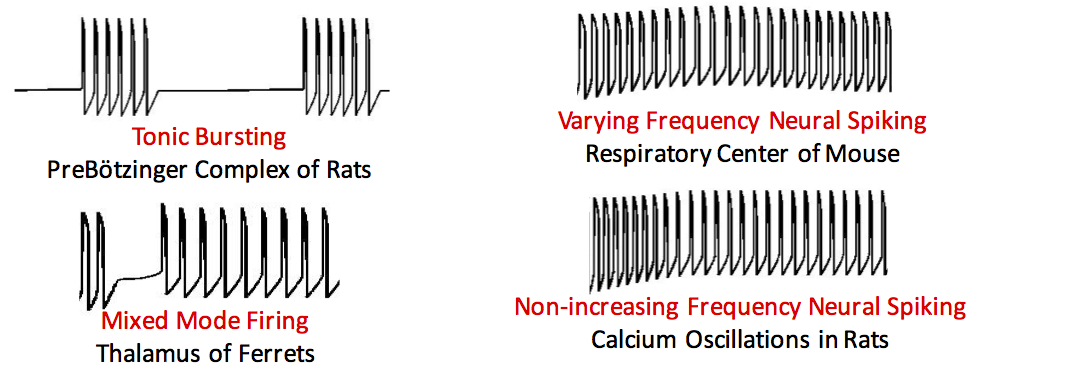
Dynamical System Paradigm that Enables Modeling Neural Activities Using Low Complexity Dynamical Systems
Three basic neuron models are popular in computational neuroscience applications. They include the Hodgkin-Huxley model, the Hindmarsh-Rose model and the FitzHugh-Nagumo model. While the FitzHugh-Nagumo model is the simplest of the three – having just two differential equations – it is unable to capture the dynamics of neuronal bursting. We have developed an innovative low-dimensional dynamical system that generates a broad range of spiking activities that were previously obtained from more complicated mathematical models. An extension is proposed for this particular model by permitting one of the parameters to vary with time. This allows for simulating neuronal spiking including tonic bursting, mixed mode firing, neuronal spiking with non-increasing frequency and spiking with varying frequency. Furthermore, a novel algorithm exploiting the structure of the model is presented for parameter estimation. This online estimation algorithm which can be used even in the absence of complete specifications for the system outperforms an Extended Kalman Filter in several different scenarios.